Abstract
Introduction: Peripheral T-cell lymphomas (PTCL) encompass rare and heterogeneous forms of aggressive non-Hodgkin lymphoma generally associated with dismal clinical outcome. Given the poor prognosis, allogeneic stem cell transplantation (allo-HSCT) has been integrated in the treatment algorithms in the relapsed/refractory setting; however poor response to salvage therapies often hampers the possibility to benefit from this potentially curative approach. We report our institutional experience on the outcome of patients with relapsed PTCL eligible to allo-HSCT in an intention to treat (ITT) analysis.
Methods and Results: We identified 108 adult patients with relapsed/refractory PTCL treated at the Istituto Nazionale Tumori from 1992 to 2016. Of these 108 patients, 22 (20%) were transplant-ineligible because of age >65 years; 86 patients (80%) were considered transplant-eligible upon failure of 1st line therapy: 48 (45%) received allo-HSCT, while 38 (35%) did not proceed to transplant. Main reason for failure to achieve transplantation was disease progression (35 patients, 92%).
We sought to determine if specific characteristics at diagnosis and/or 1st relapse were associated to the failure to achieve transplantation. In univariate analyses, patients unable to proceed to allo-HSCT at diagnosis were older (median age 56 years vs. 46 for transplanted patients; p=0.014) and more frequently had >1 extranodal site involvement (p=0.001), however none of these variables was confirmed in multivariate analysis. As expected, patients who were not transplanted had a shorter time to 1st relapse as compared to transplanted patients (p=0.002), indicating a more aggressive, chemoresistant disease.
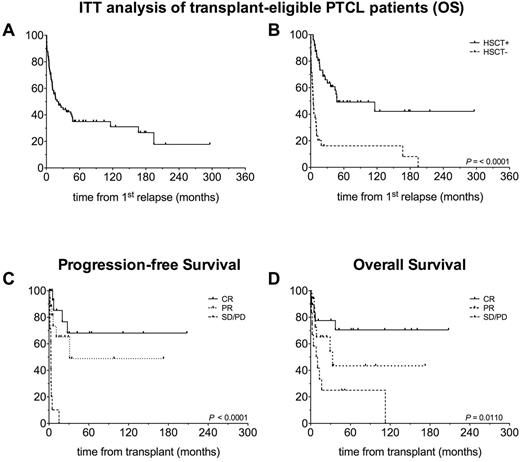
As assessed by ITT (Figure 1A and B), the estimated 5-year overall survival (OS) for transplant-eligible patients was 35%: notably survival curves significantly differed when transplanted and not transplanted patients were separated, with 5-year OS being 49% and 16%, respectively (p<0.0001).
Within the transplanted patients' cohort, clinical status at transplantation was CR in 19 patients (40%), PR in 17 (35%), SD/PD in 12 (25%). Thirty-one patients (65%) underwent a reduced intensity conditioning, 17 (35%) underwent myeloablative conditioning, with most patients (n=39, 81%) receiving a thiotepa/fludarabine/cyclophosphamide-based regimen. Donor type was matched related for 13 patients (27%), matched unrelated for 24 (50%) and haploidentical for 11 (23%). GvHD prophylaxis consisted mainly in methotrexate-cyclosporine (n=31) plus ATG in case of unrelated donor grafts. PT-Cy or ex vivo T cell depletion were employed in the haploidentical setting. With a median follow up for surviving patients of 45 months (range 2 - 208 months), the 3-year cumulative incidence of non-relapse mortality and relapse were 19% and 48%, respectively. Estimated 5-year progression-free survival (PFS) was 45%. Importantly, outcomes were more favorable in patients transplanted in CR (5-year PFS 68%, OS 70%) vs. patients transplanted in PR (5-year PFS 43%, OS 49%) or active disease (5-year PFS 0%, OS 25%; PFS: p=0.0001; OS: p=0.011) (Figure 1C and D).
Conclusions: This study supports the curative potential of allo-HSCT in relapsed PTCL patients with otherwise poor survival. Allo-HSCT can provide long-term remissions and indeed a PFS plateau appears beyond 5 years for patients transplanted in CR/PR. Unfortunately, the primary barrier to curative therapy remains achieving a sufficient response to salvage therapy and future studies should be aimed at increasing the response rate in this population.
Corradini: Roche: Honoraria; Janssen: Honoraria; Novartis: Honoraria; Takeda: Honoraria; Gilead: Honoraria; Celgene: Honoraria; Sanofi: Honoraria; Amgen: Honoraria.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal